Calcium Chloride and Water Equation
Calcium carbonate can also be an additive to food products for livestock animals and humans and as a supplement in vitamins. It is used in natural farming.

Calcium Chloride Baking Soda Water Reaction Calcium Chloride Baking Soda Baking
The component ions in a salt compound can be either inorganic such as.
. Calcium phosphate may dissolve slightly in CO 2-containing water. Gases change the color of the entire litmus strip since the whole surface is exposed. The list of uses of calcium carbonate is given below.
With molar masses of 2299 and 3545 gmol respectively 100 g of NaCl contains 3934 g Na and 6066 g Cl. Only compounds that are aqueous are split into ions. Calcium carbide compound reacts with nitrogen at higher temperatures to produce calcium cyanamide.
517 g of fertiliser was added to a 2500 mL volumetric flask and water was added to make it up to the mark. Write the equation and balance it if necessary NaClaq AgNO 3 aq AgCls NaNO 3 aq Step 2. CaC 2 N 2 CaCN 2 C.
Litmus paper may be dampened with distilled water to give a color change for a gaseous sample. Structure of Ca 3 PO 4 2. Calcium phosphate is insoluble in water but soluble in acids.
The gasliquid phase state can be plotted with a temperature-humidity diagram as shown in Fig. Uses of Calcium phosphate Ca 3 PO 4 2. Water soluble calcium phosphate is an important material for plant growth and is commonly dispersed in the soil.
It is a crystalline solid white in colour at room temperature and is highly soluble in water and hence it is hygroscopic in nature. A student used the following procedure to determine the percentage of phosphorus in a sample of water soluble fertiliser. It has a very high enthalpy change of solution and is odourless.
Neutral gases such as. 2 is based on the TH diagram of water vapor at atmospheric pressure where H AS-P101 kPa and H AS-P140 kPa indicate the equivalent moisture content of the solution at 101 kPa and 140 kPa respectively and H G-P101 kPa and H G-P140 kPa indicate the saturated moisture. Sodium chlorideaq silver nitrateaq silver chlorides sodium nitrateaq Solution.
Calcium Chloride is an ionic compound composed of chlorine and calcium. In chemistry a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions which results in a compound with no net electric charge. In a typical procedure to synthesize ACNC anhydrous calcium chloride CaCl 2 01 molL 1 gadolinium chloride hexahydrate GdCl 3 002 molL 1 and PAA 045 g were dissolved in 10.
A common example is table salt with positively charged sodium ions and negatively charged chloride ions. Sodium chloride ˌ s oʊ d i ə m ˈ k l ɔːr aɪ d commonly known as salt although sea salt also contains other chemical salts is an ionic compound with the chemical formula NaCl representing a 11 ratio of sodium and chloride ions. A particular water soluble fertiliser contains phosphorus in the form of phosphate ions PO 4 3-.
Commonly the term called nitrolime which is a calcium cyanamide can be used as fertilizer. Sodium chloride is the salt. It is also hydrolyzed to cyanamide H 2 NCN.
This compound is widely used for de-icing and dust control. It is represented by using the below equation. In water and sewer treatment plants calcium carbonate is employed in the removal of acidity and impurities.
Write the ionic equation for the word equation. Remember acids and bases refer only to aqueous water-based solutions so pH paper wont change color in non-aqueous liquids such as vegetable oil.

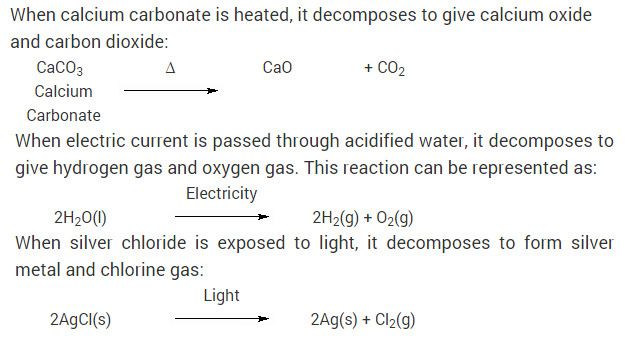
Chemical Reactions And Equation Cbse Class 10 Science Ncert Solutions Chemical Reactions Science Textbook Science

Pin By Carin Barber On Chemistry Notes Chemistry Notes Chemistry Writing

Pin By Carin Barber On Chemistry Notes Chemistry Notes Chemistry Writing

Solubility Product Constants Silver Chloride Agcl Is Rather Insoluble In Water Careful Experiments Show That If Solid Solubility Experiments Silver Chloride
0 Response to "Calcium Chloride and Water Equation"
Post a Comment